The pressure is on to make metallic hydrogen
In a few highly specialized laboratories, scientists bombard matter with the world’s most powerful electrical pulses or zap it with sophisticated lasers. Other labs squeeze heavy-duty diamonds together hard enough to crack them.
All this is in pursuit of a priceless metal. It’s not gold, silver or platinum. The scientists’ quarry is hydrogen in its most elusive of forms.
Several rival teams are striving to transform hydrogen, ordinarily a gas, into a metal. It’s a high-stakes, high-passion pursuit that sparks dreams of a coveted new material that could unlock enormous technological advances in electronics.
“Everybody knows very well about the rewards you could get by doing this, so jealousy and envy [are] kind of high,” says Eugene Gregoryanz, a physicist at the University of Edinburgh who’s been hunting metallic hydrogen for more than a decade.
Metallic hydrogen in its solid form, scientists propose, could be a superconductor: a material that allows electrons to flow through it effortlessly, with no loss of energy. All known superconductors function only at extremely low temperatures, a major drawback. Theorists suspect that superconducting metallic hydrogen might work at room temperature. A room-temperature superconductor is one of the most eagerly sought goals in physics; it would offer enormous energy savings and vast improvements in the transmission and storage of energy.
Metallic hydrogen’s significance extends beyond earthly pursuits. The material could also help scientists understand our own solar system. At high temperatures, compressed hydrogen becomes a metallic liquid — a form that is thought to lurk beneath the clouds of monstrous gas planets, like Jupiter and Saturn. Sorting out the properties of hydrogen at extreme heat and high pressure could resolve certain persistent puzzles about the gas giants. Researchers have reported brief glimpses of the liquid metal form of hydrogen in the lab — although questions linger about the true nature of the material.
While no lab has yet produced solid metallic hydrogen, the combined efforts of many scientists are rapidly closing in on a more complete understanding of the element itself — as well as better insight into the complex inner workings of solids.
Hydrogen, the first element in the periodic table and the most common element in the universe, ought to be easy to understand: a single proton paired with a single electron. “What could be more simple than an assembly of electrons and protons?” asks theoretical physicist Neil Ashcroft of Cornell University. But at high pressures, the physics of hydrogen rapidly becomes complex.
At room temperature and atmospheric pressure, hydrogen is a gas. But like other materials, altered conditions can transform hydrogen into a solid or a liquid. With low enough temperatures or a sufficiently forceful squeeze, hydrogen shape-shifts into a solid. Add heat while squeezing, and it becomes a liquid.
If subjected to still more extreme conditions, hydrogen can — at least theoretically — undergo another transformation, into a metal. All metals have one thing in common: They conduct electricity, due to free-flowing electrons that can go where they please within the material.
Squeeze anything hard enough and it will become a metal. “Pressure does a great job of dislodging the outer electrons,” Ashcroft says. This is what scientists are aiming to do with hydrogen: create a sloshing soup of roving electrons in either a liquid or a solid.
When hydrogen is compressed, many atoms begin to interact with one another, while paired in molecules of two hydrogen atoms each. The underlying physics becomes a thorny jumble. “It is amazing; the stuff takes up incredibly complex arrangements in the solid state,” says Ashcroft, the first scientist to propose, in 1968, that metallic hydrogen could be a high-temperature superconductor.
Hydrogen’s complexity fascinates scientists. “It’s not just the metallization question that’s of interest to me,” says Russell Hemley, a chemist at the Carnegie Institution for Science in Washington, D.C., and Lawrence Livermore National Laboratory in California. Studying the intricacies of hydrogen’s behavior can help scientists refine their understanding of the physics of materials.
In 1935, when physicists Eugene Wigner and Hillard Bell Huntington of Princeton University first predicted that compressed solid hydrogen would be metallic, they thought the transition to a metal might occur at a pressure 250,000 times that of Earth’s atmosphere. That may sound like a lot, but scientists have since squeezed hydrogen to pressures more than 10 times as high — and still no solid metal.
Scientists originally expected that the transition would be a simple flip to metallic behavior. Not so, says theoretical physicist David Ceperley of the University of Illinois at Urbana-Champaign. “Nature has a lot more possibilities.” Solid hydrogen exists in multiple forms, each with a different crystal structure. As the pressure climbs, the wily hydrogen molecules shift into ever-more-complex arrangements, or phases. (For physicists, the “phase” of matter goes deeper than the simple states of solid, liquid or gas.) The number of known solid phases of hydrogen has grown steadily as higher pressures are reached, with four phases now well established. The next phase scientists find could be a metal — they hope.
Story continues below timeline
Timeline: The race to make metallic hydrogen
Rival research teams are rushing to transform solid or liquid hydrogen into a metal. With each experiment, the pressure rises. They have two very different aims: a room-temperature superconductor and a window into the gas giants.
If solid metallic hydrogen turns out to be a room-temperature superconductor, it would have to be crushed to work, making it impractical for many applications. But if hydrogen could hold its metallic form after the pressure is released, as some researchers have suggested, “it would be revolutionary,” says physicist Isaac Silvera, who leads the metallic hydrogen hunt at Harvard University. Such a material could be used in electrical wires to reduce loss of energy and decrease the world’s power consumption. And it might lead to efficient, magnetically levitated trains and technological advances in nuclear fusion, supercomputing and more.
While one group of would-be metallurgists is searching for solid metal, other investigators seek the scientifically intriguing liquid hydrogen metal. Their techniques differ in timescale and size. To produce liquid metal, scientists violently slam hydrogen for fractions of a second at a time, using enormous machines at national laboratories. Scientists searching for a solid metal, on the other hand, use fist-sized devices to capture hydrogen between the tips of two tiny diamonds and slowly squeeze.
Diamonds have it rough
Crushing an ethereal, normally gaseous substance between two diamonds sounds nearly impossible. Such tricky experiments make for a field where researchers are in regular disagreement over their latest results. “We’re still missing high-quality, reliable data,” says physicist Alexander Goncharov. “The issue is the experiments are too challenging.”
In his office at the Geophysical Laboratory of the Carnegie Institution, Goncharov opens a desk drawer and pulls out a device called a diamond anvil cell. The cylinder of metal is small enough for Goncharov to cradle in his palm. Bits of precisely machined steel and tough tungsten carbide are held together with four screws. Through portals in the top and bottom, bright sparkles shimmer: diamonds.
Inside the capsule, two diamonds taper to tiny points a few hundredths of a millimeter wide. They pinch the material within, squashing it at over a million times atmospheric pressure. The gap between the minuscule anvils can be as small as a few thousandths of a millimeter, about the size of a human red blood cell.
Once they’ve been pressurized, diamond anvil cells will hold the pressure almost indefinitely. The prepared cells can be carried around — inspected in the laboratory, transported to specialized facilities around the world — or simply stored in a desk drawer. Goncharov regularly travels with them. (Tip from the itinerant scientist: If questions arise at airport security, “never use the word ‘diamonds.’ ”) The diamond anvil can squeeze more than hydrogen — materials from iron to sodium to argon can be crushed in the diamond vise.
To identify new, potentially metallic phases of solid hydrogen within the pressurized capsules, scientists shine laser light onto the material, measuring how molecules vibrate and rotate — a technique called Raman spectroscopy (SN: 8/2/08, p. 22). If a new phase is reached, molecules shift configurations, altering how they jiggle. Certain types of changes in how the atoms wobble are a sign that the new phase is metallic. If the material conducts electricity, that’s another dead giveaway. A final telltale sign: The normally translucent hydrogen should acquire a shiny, reflective surface.
Significant hurdles exist for diamond anvil cell experiments. Diamonds, which cost upwards of $600 a pop, can crack under such intense pressures. Hydrogen can escape from the capsule, or diffuse into the diamonds, weakening them. So scientists coat their diamonds with thin layers of protective material. The teams each have their own unique recipe, Goncharov says. “Of course, everyone believes that their recipe is the best.”
Three phases of solid hydrogen have been known since the late 1980s. With the discovery of a fourth phase in 2011, “the excitement was enormous,” Gregoryanz says. In Nature Materials, Mikhail Eremets and Ivan Troyan at the Max Planck Institute for Chemistry in Mainz, Germany, reported that a new phase appeared when they squashed room-temperature hydrogen to over 2 million times atmospheric pressure. Goncharov, Gregoryanz and colleagues created the new phase and deduced its structure in Physical Review Letters in 2012. In phase IV, as it’s known, hydrogen arranges itself into thin sheets — somewhat like the single-atom-thick sheets of carbon known as graphene, the scientists wrote.
Progress doesn’t come easy. With each new paper, scientists disagree about what the results mean. When Eremets and colleagues discovered the fourth phase, they thought they also had found metallic hydrogen (SN: 12/17/11, p. 9). But that assertion was swiftly criticized, and it didn’t stand up to scrutiny.
The field has been plagued by hasty claims. “If you look at the literature for the last 30 years,” Gregoryanz says, “I think every five years there is a claim that we finally metallized hydrogen.” But the claims haven’t been borne out, leaving scientists perpetually skeptical of new results.
In a recent flurry of papers, scientists have proposed new phases — some that might be metallic or metal-like precursors to a true metal — and they are waiting to see which claims stick. Competing factions have volleyed papers back and forth, alternately disagreeing and agreeing.
A paper from Gregoryanz’s group, published in Nature in January, provided evidence for a phase that was enticingly close to a metal (SN Online: 1/6/16), at more than 3 million times atmospheric pressure. But other scientists disputed the evidence. In their own experiments, Eremets and colleagues failed to confirm the new phase. In a paper posted online at arXiv.org just days after Gregoryanz’s paper was published, Eremets’ team unveiled hints of a “likely metallic” phase, which occurred at a different temperature and pressure than Gregoryanz’s new phase.
A few months later, Silvera’s group squeezed hydrogen hard enough to make it nearly opaque, though not reflective — not quite a metal. “We think we’re just below the pressure that you need to make metallic hydrogen,” Silvera says. His findings are consistent with Eremets’ new phase, but Silvera disputes Eremets’ speculations of metallicity. “Every time they see something change they call it metallic,” Silvera says. “But they don’t really have evidence of metallic hydrogen.”
All this back and forth may seem chaotic, but it’s also a sign of a swiftly progressing field, the researchers say. “I think it’s very healthy competition,” Gregoryanz says. “When you realize that somebody is getting ahead of you, you work hard.”
The current results are “not very well consistent, so everybody can criticize each other,” Eremets says. “For me it’s very clear. We should do more experiments. That’s it.”
There are signs of progress. In 2015, Eremets and colleagues discovered a record-breaking superconductor: hydrogen sulfide, a compound of hydrogen and sulfur. When tightly compressed into solid form, hydrogen sulfide superconducts at temperatures higher than ever seen before: 203 kelvins (−70° Celsius) (SN: 8/8/15, p. 12).
Adding sulfur to the mix stabilizes and strengthens the hydrogen structure but doesn’t contribute much to its superconducting properties. Hydrogen sulfide is so similar to pure hydrogen, Eremets says, that, “in some respects we already found superconductivity in metallic hydrogen.”
Brief glimmers
Giant, gassy planets are chock-full of hydrogen, and the element’s behavior under pressure could explain some of these planets’ characteristics. A sea of flowing liquid hydrogen metal may be the source of Jupiter’s magnetic field (SN: 6/25/16, p. 16). Learning more about metallic hydrogen’s behavior deep inside such planets could also help resolve a long-standing puzzle regarding Saturn: The ringed behemoth is unexpectedly bright. The physics of hydrogen’s interactions with helium inside the planet could provide the explanation.
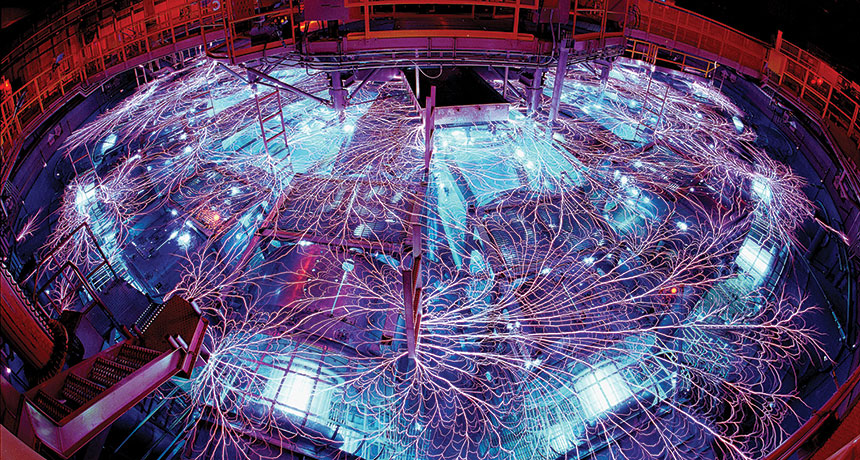
Using a radically different set of technologies, a second band of scientists is on the hunt for such liquid metallic hydrogen. These researchers have gone big, harnessing the capabilities of new, powerful machines designed for nuclear fusion experiments at government-funded national labs. These experiments show the most convincing evidence of metallic behavior so far — but in hydrogen’s liquid, not solid, form. These enormous machines blast hydrogen for brief instants, temporarily sending pressures and temperatures skyrocketing. Such experiments reach searing temperatures, thousands of kelvins. With that kind of heat, metallic hydrogen appears at lower, more accessible pressures.
Creating such conditions requires sophisticated equipment. The Z machine, located at Sandia National Laboratories in Albuquerque, generates extremely intense bursts of electrical power and strong magnetic fields; for a tiny instant, the machine can deliver about 80 terawatts (one terawatt is about the total electrical power–generating capacity in the entire United States).
A group of scientists recently used the Z machine to launch a metal plate into a sample of deuterium — an isotope of hydrogen with one proton and one neutron in its nucleus — generating high pressures that compressed the material. The pummeled deuterium showed reflective glimmers of shiny metal, the scientists reported last year in Science. “It starts out transparent, it goes opaque, and then later we see this reflectivity increase,” says Marcus Knudson of Sandia and Washington State University in Pullman.
Another group is pursuing a different tactic, using some of the most advanced lasers in the world, at the National Ignition Facility at Lawrence Livermore. Scientists there zap hydrogen to produce high pressures and temperatures. Though the conclusions of this experiment are not yet published, says physicist Gilbert Collins of Lawrence Livermore, one of the leaders of the experiment, “we have some really beautiful results.”
The first experiment to show evidence of liquid metallic hydrogen was performed at Lawrence Livermore in the 1990s. A team of physicists including William Nellis, now at Harvard, used a sophisticated gunlike apparatus to shoot projectiles into hydrogen at blisteringly fast speeds. The resulting hydrogen briefly conducted electricity (SN: 4/20/96, p. 250).
These experiments face hurdles of their own — it’s a struggle to measure temperature in such systems, so scientists calculate it rather than measuring it directly. But many researchers are still convinced by these results. Metallic hydrogen “certainly has been produced by shock techniques,” Cornell’s Ashcroft says.
Some scientists still have questions. “It’s certainly difficult to tell if something is a metal or not at such high temperature,” Collins says. Although they need high temperatures to reach the metal liquid phase, some physicists define a metal based on its behavior at a temperature of absolute zero. Current experiments hit high pressures at temperatures as close to zero as possible to produce relatively cool liquid metallic hydrogen.
Scientists who conduct palm-sized diamond anvil cell experiments refuse to be left out of the liquid-metal action. They’ve begun to use laser pulses to heat and melt hydrogen crammed into the cells. The results have stirred up new disagreements among competing groups. In April’s Physical Review B, Silvera and coauthors reported forming liquid metallic hydrogen. But under similar conditions, Goncharov and others found only semiconducting hydrogen, not a metal. They reported their results in Physical Review Letters in June.
“There’s kind of a crisis now with these different experiments,” says Illinois theorist Ceperley. “And there’s a lot of activity trying to see who’s right.” For now, scientists will continue refining their techniques until they can reach agreement.
The major players have managed to reach consensus before. Four phases of solid hydrogen are now well established, and researchers agree on certain conditions under which solid hydrogen melts.
Solid metallic hydrogen, however, has perpetually seemed just out of reach, as theoretical predictions of the pressure required to produce it have gradually shifted upward. As the goalposts have moved, physicists have reached further, achieving ever-higher pressures. Current theoretical predictions put the metal tantalizingly close — perhaps only an additional half a million times atmospheric pressure away.
The quest continues, propelled by a handful of hydrogen-obsessed scientists.
“We all love hydrogen,” Collins says. “It has the essence of being simple, so that we think we can calculate something and understand it, while at the same time it has such a devious nature that it’s perhaps the least understandable material there is.”