Chinese scientists make breakthrough in BCI-assisted rehabilitation trial, 'showing higher safety than Musk's Telepathy'
China's leading Tsinghua University announced on Wednesday that Chinese scientists had made a breakthrough in the world's first patient brain-computer interfaces (BCI) rehabilitation trial on Monday, one day after Tesla CEO Elon Musk announced the success of a procedure to implant Neuralink's brain chip into the first human patient.
The Chinese scientists' research has met the highest standards of safety and their achievements are expected to be commercially available within the next two years, scientists involved in the research told the Global Times on Wednesday.
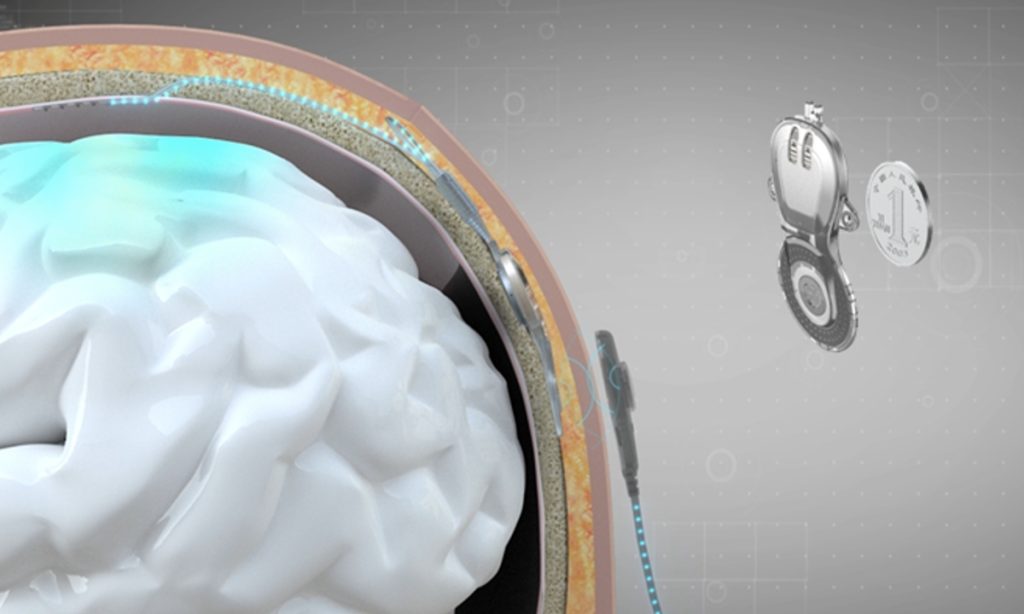
The Global Times learned from the university that a team, led by principal Biomedical Engineering researcher Hong Bo from the School of Medicine with Tsinghua University, designed and developed the wireless minimally invasive implanted BCI technology device NEO (Neural Electronic Opportunity). The NEO was successfully implanted into a patient's brain for the BCI-assisted treatment trial at the Xuanwu Hospital in Beijing, on October 24, 2023.
The subject of the trial is a 54-year-old male patient with complete spinal cord injury of the cervical spine after a car accident. He has been paralyzed for 14 years in all four limbs since then.
After implanting two coin-sized BCI processors into his brain, scientists successfully collected intracranial neural signals of the somatosensory motor brain area of the patient.
Ten days after the surgery, the patient was discharged and returned home. When used at home, the external device of the NEO supplies power to the internal device through the scalp and receives neural signals from the brain, which are then transmitted to a computer or mobile phone through decoding algorithms to achieve BCI communication.
This system uses near-field wireless power supply and communication technology. The implanted internal device in the skull does not require a battery and can be used for a lifetime, the Global Times learned.
After three months of home-based BCI rehabilitation training, the patient was able to use brainwave activity to drive an air-powered glove and drink water independently, with a decoding accuracy rate of over 90 percent.
The American Spinal Injury Association (ASIA) clinical score and somatosensory evoked potential response of the patient's spinal cord injury has also significantly improved. Pictures and videos provided by Tsinghua University show the first patient successfully initiating brain-controlled grasping of a mineral water bottle through the wireless minimally invasive BCI.
One day before the Chinese team's research was revealed, Musk said that his BCI company, Neuralink, had successfully completed the first human brain device implantation surgery. Although the surgery was a success, Musk's invasive BCI technology experiment has sparked controversy in terms of surgical safety and medical ethics in the US.
The Chinese research team told the Global Times that their project, which is different from Neuralink's "mind control" device Telepathy, has achieved two major breakthroughs in wireless minimally invasive BCI technology.
On one hand, the NEO is implanted, burying the internal device in the skull, with electrodes covering the dura mater between the skull and the cerebral cortex, which protects neural tissue. It ensures the quality of intracranial signals without damaging neural tissue. On the other hand, it uses near-field wireless power supply and signal transmission. The implanted internal device in the skull does not require a battery and can be used for a lifetime.
Team leader Hong told the Global Times in an exclusive interview that compared with Neuralink's technology, the NEO technology has the advantages of higher safety and long-term use.
Currently, BCI technology is classified into three categories: Non-invasive, invasive, and semi-invasive, based on whether it requires the invasion of the brain and the degree of invasion, according to Hong.
Hong explained that invasive BCI usually involves the implantation of a large number of neural electrodes in the cerebral cortex, which causes significant trauma and makes it difficult to solve the problem of immune-inflammatory reactions. After a certain period of implantation, the electrodes will be covered by glial cells, resulting in a gradual decrease in signal quality.
Conversely, wireless minimally invasive implanted BCI technology is usually implanted on the human dura mater, without invading the neural cells of the cerebral cortex. It uses a combination of software and hardware to enhance signal quality, effectively solving the problem of biocompatibility and achieving a balance between high signal intensity and minimal implantation damage.
The Global Times learned from the team that the clinical trial of this wireless minimally invasive BCI was approved by the Xuanwu Hospital in April 2023. It has also been registered for both international and domestic clinical trials of implanted medical devices.
The second clinical surgery for a patient with spinal cord injury was successfully performed by Professor Jia Wang's team at the Tiantan Hospital on December 19, 2023. The patient is currently undergoing home-based rehabilitation training.
Asked when the NEO technology could be available on the market, Hong told the Global Times that currently, the technology is undergoing large-scale clinical trials in accordance with the relevant regulations. It can be put into actual application after obtaining an implanted medical devices license.
"It is expected to take at least two years," he said.